While helping my dad fix our car, I found out that understanding the aluminum number of neutrons is crucial for making strong car parts. It amazed me how these tiny particles play a big role in the metal’s strength.
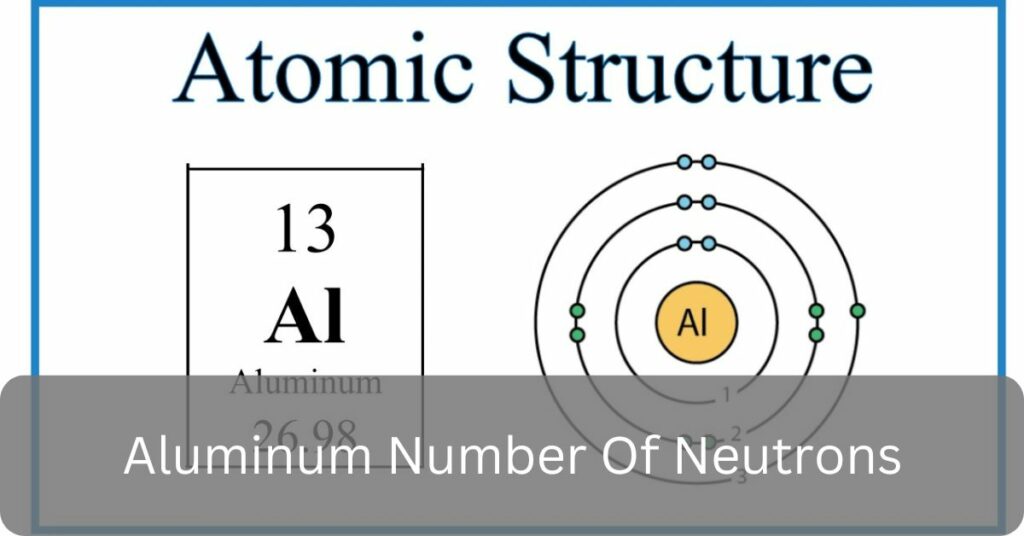
Aluminum’s atomic number of 13 means it has 13 protons, and its most common form, aluminum-27, has 14 neutrons, highlighting the importance of understanding the ‘aluminum number of neutrons.
In this article, we’ll discuss the aluminum number of neutrons. This helps us understand more about aluminum and how it’s used in different things. Let’s explore why knowing about neutrons in aluminum is important.
What Is Aluminum – Must Know!
Firstly, let’s understand what aluminum is. Aluminum is a type of metal that’s lightweight, strong, and rust-resistant.
It’s used in many things we see and use every day, like cans, foil, and even parts of aeroplanes. Aluminum is also easy to shape and recycle, making it a popular choice in various industries.
What Are Neutrons – Play A Big Role!
Neutrons are tiny particles found in the nucleus of atoms, alongside protons. Unlike protons, which have a positive electrical charge, neutrons are electrically neutral, meaning they have no charge.
Neutrons play an important role in determining the stability and behaviour of atoms, especially in deciding what type of element it is.
Why Are Neutrons Important In Aluminum – Let’s Find Out Together!
1. Determining Stability:
Neutrons are like helpers inside aluminum atoms, balancing out the positive charge from protons. This balancing act helps keep aluminum atoms stable, preventing them from breaking apart easily.
2. Influencing Isotopes:
Different types of aluminum, called isotopes, have different numbers of neutrons. These extra neutrons change how heavy the aluminum is and what it can be used for. Some isotopes might be better for making things like cans, while others might be better for making strong parts for aeroplanes.
3. Enhancing Strength:
Neutrons also play a role in making aluminum strong and tough. They help create a sturdy structure inside aluminum atoms, which makes the metal resistant to bending and breaking. This strength makes aluminum useful for building things like cars, aeroplanes, and even buildings.
4. Enabling Alloy Formation:
Aluminum can mix with other metals to make alloys, which are even stronger and more useful. Neutrons help in this mixing process, making sure the atoms of aluminum and other metals stick together properly. This allows us to create alloys with specific properties needed for different purposes.
5. Supporting Research:
Scientists study aluminum and its neutrons to learn more about how it works and how we can make it better.
By understanding how neutrons affect aluminum, researchers can develop new materials and technologies, leading to improvements in various fields like transportation, construction, and manufacturing.
How Many Neutrons Does Aluminum Have – Don’t Miss Out!
Aluminum usually has 14 neutrons. We figure this out by looking at its most common form, aluminum-27. The atomic number for aluminum is 13.
We subtract 13 from 27 to find the number of neutrons. Knowing the “aluminum number of neutrons” helps us understand more about aluminum and its uses.
Why Does Aluminum Have Different Neutron Counts – Expand Your Knowledge!
Different forms of aluminum, known as isotopes, can have varying numbers of neutrons. For example, the most common isotope, aluminum-27(denoted as 27Al), contains 14 neutrons.
However, other isotopes like aluminum-26 (26 Al) or aluminum-28 (28 Al) may have different neutron counts.
These isotopes occur naturally and have different proportions in the environment. The presence of different isotopes with varied neutron counts contributes to the diversity of aluminium’s atomic structure, influencing its overall properties and behaviour.
How Does Aluminum Number Of Neutrons Help – A Lot Of Things!
Understanding how many neutrons aluminum has is really important. First, it helps us figure out how stable aluminum is.
This matters a lot in things like building materials and nuclear science. Also, the number of neutrons affects how strong aluminum is and what we can use it for.
It’s crucial for making sure the stuff we make with aluminum, like cars and airplanes, is safe and works well.
Plus, by studying Aluminum Number Of Neutrons, scientists can find new ways to make better materials and technology. So, knowing about Aluminum Number Of Neutrons is super helpful for lots of things!
How Does The Number Of Neutrons Change The Weight Of Different Types Of Aluminum?
The number of neutrons in different types of aluminum isotopes affects their weight. Isotopes with more neutrons tend to be heavier than those with fewer neutrons because neutrons contribute to the overall mass of the atom.
For example, aluminum-27, which has 14 neutrons, is heavier than aluminum-26, which has 13 neutrons. Therefore, the number of neutrons directly influences the atomic mass of aluminum isotopes, ultimately affecting their weight.
How Do Neutrons Affect Whether Aluminum Is Stable Or Not – Here To Know!
Neutrons are important for keeping aluminum stable. They balance out the positive charge from protons in the atom’s center. When there’s the right balance of neutrons and protons, aluminum stays stable.
But if there are too few or too many neutrons, the atom becomes less stable. This balance is important for making sure aluminum works well in things like cars and buildings.
What Tools Do Scientists Use To Count The Neutrons In Aluminum?
Scientists use different ways to count the neutrons in aluminum. One way is by making aluminum samples radioactive with a method called neutron activation analysis. They then measure the radiation to find out how many neutrons there are.
Another way is mass spectrometry, where they separate aluminum isotopes based on their weight and charge. This helps them figure out the number of neutrons in each isotope.
They also use techniques like neutron scattering, which gives them clues about the arrangement of atoms and indirectly helps count the neutrons in aluminum.
Frequently Asked Questions:
1. Can the number of neutrons in aluminum change over time?
The number of neutrons in aluminum usually stays the same unless something special happens, like radioactive decay or nuclear reactions. Sometimes, though, aluminum can change its neutron count if certain conditions are met.
2. Do all aluminum types have the same number of neutrons in every sample?
The average number of neutrons in a certain type of aluminum usually doesn’t change. However, sometimes, individual samples might have a bit more or less neutrons because of natural differences or things in the environment that affect how aluminum is made up.
3. Can scientists change the number of neutrons in aluminum?
Scientists can change the number of neutrons in aluminum by making aluminum atoms soak up more neutrons or by bombarding them with neutrons to make them react. These methods are used in science to study atoms and in making new materials.
4. Are there any useful uses for aluminum with specific neutron counts?
Yes, certain types of aluminum with specific neutron counts have special uses, especially in fields like nuclear energy and medicine. They’re used in things like fuel or in making medical equipment for scanning the body.
Conclusion:
Understanding Aluminum Number Of Neutrons is important. It helps us figure out how aluminum atoms work and affect things around us. This knowledge helps us make better materials and technology using aluminum, leading to discoveries and improvements in various areas.