Whenever I think about chemistry, I wonder, How many electrons does Cl have Learning about it helps me understand how things stick together and make me curious about the stuff around me.
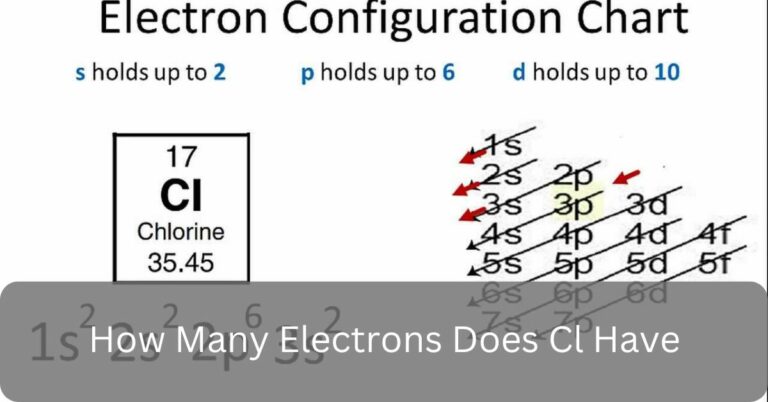
In chemistry, it’s important to know about basic elements like chlorine (Cl). One common inquiry is, How many electrons does Cl have Well, with an atomic number of 17, chlorine boasts 17 electrons in total, with 7 occupying its outermost shell, dictating its chemical behaviour.
This article, talks about something cool in chemistry: How many electrons does Cl have? Let’s explore the atomic structure of chlorine and find out how many little particles chlorine packs around its nucleus. Get ready for some fascinating insights!
What Are Electrons – Tiny Messengers!

Electrons are tiny, negatively charged particles that zoom around the nucleus of an atom. They’re like little messengers of electricity, buzzing around the centre of the atom in specific paths called energy levels or shells. Electrons are super important because they determine how atoms interact with each other in chemical reactions.
What Is Chlorine (Cl) – Let’s explore!
However, Chlorine (Cl) is a chemical element found on the periodic table with the symbol Cl and the atomic number 17. It’s a greenish-yellow gas in its natural state and is highly reactive.
Chlorine is widely used in industry, water treatment, and as a disinfectant. It’s also a key component in many household cleaning products.
How Many Electrons Does Cl Have – Stay informed!
As I mentioned before, Chlorine (Cl) has 17 electrons. This number corresponds to its atomic number, which is 17 as well. Each electron orbits the nucleus of the chlorine atom, contributing to its overall chemical behavior and properties.
Why Does Cl Have 17 Electrons – uncover the reasons behind!
Chlorine (Cl) has 17 electrons due to its atomic number, which is 17. In atoms, the number of electrons matches the number of protons in the nucleus.
This balance ensures that the atom remains electrically neutral, with the negative charge of the electrons offsetting the positive charge of the protons.
How Does Cl Form Bonds – Get ready to learn!

The Chlorine (Cl) forms bonds by sharing or transferring electrons with other atoms. Typically, chlorine seeks to gain one electron to complete its outermost energy level and achieve a stable electron configuration, similar to that of noble gases.
This electron-sharing or transfer process allows chlorine to form bonds with various elements, resulting in the creation of compounds such as sodium chloride (table salt) and hydrogen chloride (a component of hydrochloric acid).
What Happens When Cl Gains an Electron – Don’t Miss Out!
When chlorine (Cl) gains an electron, it forms the chloride ion (Cl^-). This additional electron fills the outermost energy level of the chlorine atom, achieving a stable electron configuration with a full octet, similar to that of noble gases.
As a result, the chloride ion carries a negative charge due to the extra electron, making it attracted to positively charged ions or molecules in chemical reactions.
Why Is Chlorine Considered A Highly Reactive Element – Reactivity Impacts The World!
Chlorine is considered a highly reactive element due to its incomplete outer electron shell. With seven electrons in its outer shell, chlorine is just one electron short of achieving a stable configuration.
To fill this gap, chlorine readily reacts with other elements by gaining an electron, forming chloride ions, or by sharing electrons to complete its outer shell.
This reactivity makes chlorine an key component in many chemical processes and contributes to its widespread industrial and biological applications.
What Role Do Valence Electrons Play In Chemical Bonding?
Valence electrons play a crucial role in chemical bonding as they determine how atoms interact with each other. These electrons are found in the outermost energy level of an atom and are involved in forming chemical bonds.
Atoms tend to gain, lose, or share electrons to achieve a stable electron configuration, typically by filling or emptying their outer energy level.
This process allows atoms to form bonds with other atoms, resulting in the creation of molecules or compounds with unique chemical properties.
How does chlorine’s reactivity contribute to its various industrial and household applications?

Chlorine’s reactivity contributes to its various industrial and household applications in several ways:
1. Disinfection:
Firstly,Chlorine’s ability to react with and destroy harmful microorganisms makes it a valuable disinfectant. It is widely used in water treatment facilities to kill bacteria, viruses, and other pathogens in drinking water, swimming pools, and wastewater.
2. Bleaching:
Additionally, Chlorine’s strong oxidizing properties make it effective for bleaching processes in industries such as paper and textile manufacturing. It is used to remove color from materials and produce white or light-colored products.
3. Chemical Synthesis:
Moreover, Chlorine is a key ingredient in the production of a wide range of chemicals, including PVC (polyvinyl chloride), solvents, pesticides, and pharmaceuticals. Its reactivity allows it to participate in various chemical reactions, enabling the synthesis of these important compounds.
4. Plastics Production:
Furthermore, it is used in the production of PVC, one of the most widely used plastics in the world. PVC is used in construction, packaging, electrical insulation, and many other applications due to its durability, versatility, and cost-effectiveness.
5. Sanitization and Cleaning:
Additionally, Chlorine-based compounds, such as bleach (sodium hypochlorite), are commonly used in household cleaning products to disinfect surfaces, remove stains, and eliminate odors.
6. Chemical Manufacturing:
Lastly, Chlorine’s reactivity facilitates the production of chlorinated chemicals, which are used as intermediates in the synthesis of various industrial and consumer products, including solvents, refrigerants, and plastics.
Frequently Asked Questions:
1. What happens when Cl gains or loses electrons?
When chlorine gains an electron, it forms the chloride ion (Cl^-), achieving a stable electron configuration. Conversely, when it loses an electron, it forms the cation Cl^+, which is less common due to chlorine’s high electronegativity.
2. Can chlorine have a different number of electrons in various chemical compounds?
While chlorine typically has 17 electrons in its neutral state, it can form compounds where its electron count may vary due to bonding with other elements.
3. How does the electron configuration of chlorine impact its chemical properties?
The arrangement of electrons in chlorine’s energy levels influences its reactivity and ability to form bonds with other elements, impacting its role in chemical reactions and compounds.
4. Is the electron count of chlorine consistent across all isotopes?
Although the number of protons in a chlorine atom remains constant at 17, different isotopes may have varying numbers of neutrons, affecting their overall mass but not necessarily their electron count.
5. What happens when chlorine loses multiple electrons?
While chlorine typically gains electrons to achieve stability, under certain conditions, it can lose multiple electrons, forming unusual cations with positive charges, which are less common compared to its more stable chloride ion form.
Conclusion:
knowing how many electrons chlorine (Cl) has is really important in chemistry. With 17 electrons in its neutral form, chlorine’s behavior in reactions and its ability to form ions become clearer.
Understanding How Many Electrons Does Cl Have helps us see why chlorine is so useful in many everyday things, from cleaning products to water treatment, making it a vital part of our lives.